
LAB3244
CMVPCR
CMVPCRbuccal
CMVPCRUR
| Specimen Source | |
|---|---|
|  Minimum volume (non-urine): >0.2 mL Minimum volume (urine): >0.3 mL |
| 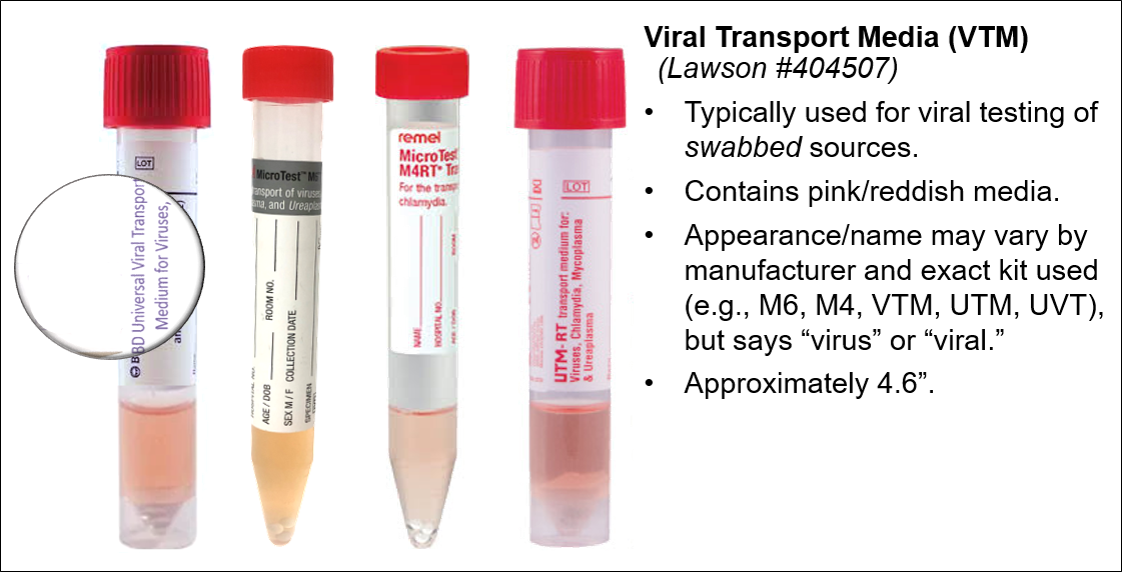 |
Internal: Ambient
Offsite: Refrigerated. Do not freeze urine.
Buccal swabs and Urine (CMVPCRbuccal and CMVPCRUR): Day shift: Daily
Other sources (CMVPCR): Day shift: Tuesday, Thursday
Buccal (CMVPCRbuccal):
Room Temp: 24 hours
Refrigerated: 3 days
Frozen: 6 months
Urine (CMVPCRUR):
Room Temp: 24 hours
Refrigerated: 3 days
Frozen: Unacceptable
Other Sources (CMVPCR):
Room Temp: 12 hours
Refrigerated: 3 days
Frozen: ≥31 days
Specimen source must be identified on order or requisition.
Quantitative results will be reported for CSF, BAL, bronch wash, tracheal aspirate, amniotic fluid, and eye fluid.
Qualiative results will be given for urine, buccal/oral swabs, stool, or diluted samples.
Buccal swabs and Urine (CMVPCRbuccal and CMVPCRUR): Day shift: Daily
Other sources (CMVPCR): Day shift: Tuesday, Thursday
LAB3244
CMVPCR
CMVPCRbuccal
CMVPCRUR
Buccal (CMVPCRbuccal):
Component Result Comment (buccal):
CMV Not Detected
An interpretation of “Not Detected” does not rule out the presence of inhibitors or CMV DNA concentration below the limit of detection of the assay.
Result Comment (buccal):
The CMV buccal swab assay is a qualitative nucleic acid amplification test for the detection of CMV using the cobas 6800 system. This assay is based on the in vitro CMV nucleic acid amplification test and has been modified for use with buccal swab specimens. The analytical performance characteristics were established by the UCHealth Molecular Diagnostics Laboratory. The specimen type has not been cleared or approved by the U.S. Food and Drug Administration. This test was performed in a CLIA-certified laboratory and is intended for clinical use.
Urine (CMVPCRUR):
Interpretation (urine):
Not detected = No virus detected.
Detected = Virus detected.
Methodology (urine):
This CMV DNA real-time assay utilizes the Roche cobas 6800 CMV test. A detected result should be coupled with clinical indicators for diagnosis. A not detected result for this assay does not exclude Cytomegalovirus involvement in a disease process. This test was developed and its performance characteristics determined by the UCH Clinical Laboratory. It has not been cleared or approved by the U.S. Food and Drug Administration. The FDA has determined that such clearance or approval is not necessary. This test is used for clinical purposes. It should not be regarded as investigational or for research. This laboratory is certified under the Clinical Laboratory Improvement Amendment of 1988 ("CLIA") as qualified to perform high complexity clinical testing.
Other Sources (CMVPCR):
Interpretation:
Not detected = No virus detected.
Detected = Virus detected.
<1,000 IU/mL = Virus detected below the minimum quantitative range.
1,000 IU/mL to 1,000,000 IU/mL = Virus detected within quantitative range.
>1,000,000 IU/mL = Virus detected above maximum quantitative range.
Methodology:
This test employs real-time PCR amplification of a Cytomegalovirus-specific conserved genetic target. A detected result should be coupled with clinical indicators for diagnosis. A not detected result for this assay does not exclude Cytomegalovirus involvement in a disease process. This test was developed and its performance characteristics determined by the UCH Clinical Laboratory. It has not been cleared or approved by the U.S. Food and Drug Administration. The FDA has determined that such clearance or approval is not necessary. This test is used for clinical purposes. It should not be regarded as investigational or for research. This laboratory is certified under the Clinical Laboratory Improvement Amendment of 1988 ("CLIA") as qualified to perform high complexity clinical testing.
87497 (quantitative)
87496 (qualitative)
| Collection |
LAB3244
CMVPCR
CMVPCRbuccal
CMVPCRUR
| Specimen Source | |
|---|---|
|  Minimum volume (non-urine): >0.2 mL Minimum volume (urine): >0.3 mL |
|  |
Internal: Ambient
Offsite: Refrigerated. Do not freeze urine.
Buccal swabs and Urine (CMVPCRbuccal and CMVPCRUR): Day shift: Daily
Other sources (CMVPCR): Day shift: Tuesday, Thursday
Buccal (CMVPCRbuccal):
Room Temp: 24 hours
Refrigerated: 3 days
Frozen: 6 months
Urine (CMVPCRUR):
Room Temp: 24 hours
Refrigerated: 3 days
Frozen: Unacceptable
Other Sources (CMVPCR):
Room Temp: 12 hours
Refrigerated: 3 days
Frozen: ≥31 days
Specimen source must be identified on order or requisition.
Quantitative results will be reported for CSF, BAL, bronch wash, tracheal aspirate, amniotic fluid, and eye fluid.
Qualiative results will be given for urine, buccal/oral swabs, stool, or diluted samples.
| Ordering |
Buccal swabs and Urine (CMVPCRbuccal and CMVPCRUR): Day shift: Daily
Other sources (CMVPCR): Day shift: Tuesday, Thursday
LAB3244
CMVPCR
CMVPCRbuccal
CMVPCRUR
| Result Interpretation |
Buccal (CMVPCRbuccal):
Component Result Comment (buccal):
CMV Not Detected
An interpretation of “Not Detected” does not rule out the presence of inhibitors or CMV DNA concentration below the limit of detection of the assay.
Result Comment (buccal):
The CMV buccal swab assay is a qualitative nucleic acid amplification test for the detection of CMV using the cobas 6800 system. This assay is based on the in vitro CMV nucleic acid amplification test and has been modified for use with buccal swab specimens. The analytical performance characteristics were established by the UCHealth Molecular Diagnostics Laboratory. The specimen type has not been cleared or approved by the U.S. Food and Drug Administration. This test was performed in a CLIA-certified laboratory and is intended for clinical use.
Urine (CMVPCRUR):
Interpretation (urine):
Not detected = No virus detected.
Detected = Virus detected.
Methodology (urine):
This CMV DNA real-time assay utilizes the Roche cobas 6800 CMV test. A detected result should be coupled with clinical indicators for diagnosis. A not detected result for this assay does not exclude Cytomegalovirus involvement in a disease process. This test was developed and its performance characteristics determined by the UCH Clinical Laboratory. It has not been cleared or approved by the U.S. Food and Drug Administration. The FDA has determined that such clearance or approval is not necessary. This test is used for clinical purposes. It should not be regarded as investigational or for research. This laboratory is certified under the Clinical Laboratory Improvement Amendment of 1988 ("CLIA") as qualified to perform high complexity clinical testing.
Other Sources (CMVPCR):
Interpretation:
Not detected = No virus detected.
Detected = Virus detected.
<1,000 IU/mL = Virus detected below the minimum quantitative range.
1,000 IU/mL to 1,000,000 IU/mL = Virus detected within quantitative range.
>1,000,000 IU/mL = Virus detected above maximum quantitative range.
Methodology:
This test employs real-time PCR amplification of a Cytomegalovirus-specific conserved genetic target. A detected result should be coupled with clinical indicators for diagnosis. A not detected result for this assay does not exclude Cytomegalovirus involvement in a disease process. This test was developed and its performance characteristics determined by the UCH Clinical Laboratory. It has not been cleared or approved by the U.S. Food and Drug Administration. The FDA has determined that such clearance or approval is not necessary. This test is used for clinical purposes. It should not be regarded as investigational or for research. This laboratory is certified under the Clinical Laboratory Improvement Amendment of 1988 ("CLIA") as qualified to perform high complexity clinical testing.
| Administrative |
87497 (quantitative)
87496 (qualitative)