
LAB3249
HSVPCR
| Specimen Source | |
|---|---|
| 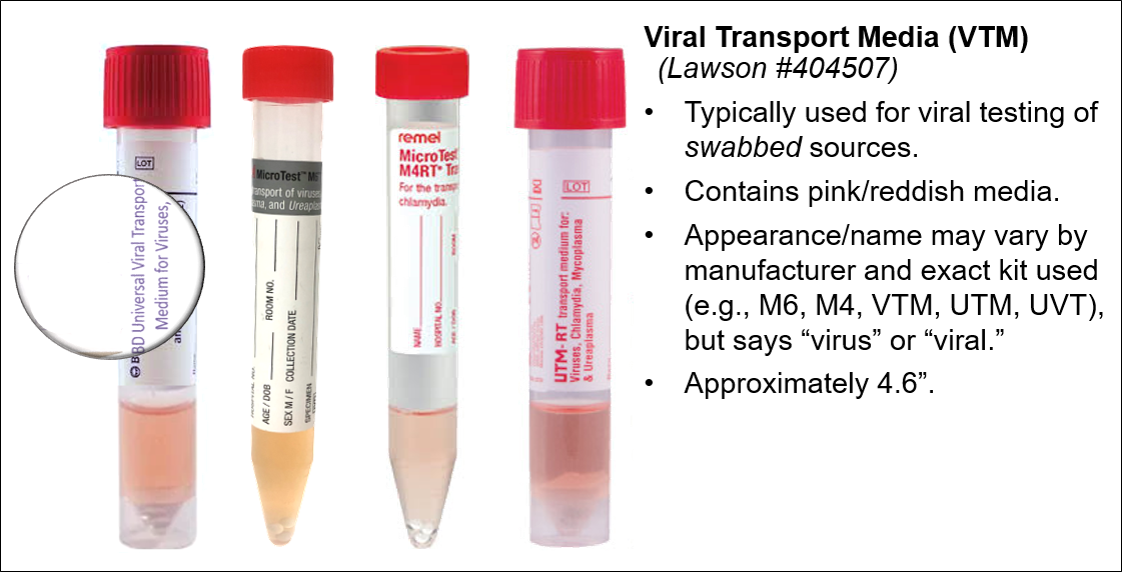 |
|  Minimum volume: >0.2 mL |
Internal: Ambient
Offsite: Refrigerated
Room Temp: 12 hours
Refrigerated: 3 days
Frozen: ≥31 days
LAB3249
HSVPCR
| HSV Type 1 | Not detected |
| HSV Type 2 | Not detected |
Interpretation:
Not detected = Negative, no virus detected.
Detected = Positive, virus detected.
Methodology:
This test employs real-time PCR amplification of a Herpes simplex virus-specific conserved genetic target. A positive result should be coupled with clinical indicators for diagnosis. A "Not detected" result for this assay does not exclude Herpes simplex virus involvement in a disease process. This test was developed and its performance characteristics determined by the UCH Clinical Laboratory. It has not been cleared or approved by the U.S. Food and Drug Administration. The FDA has determined that such clearance or approval is not necessary. This test is used for clinical purposes. It should not be regarded as investigational or for research. This laboratory is certified under the Clinical Laboratory Improvement Amendment of 1988 ("CLIA") as qualified to perform high complexity clinical testing.
| Collection |
LAB3249
HSVPCR
| Specimen Source | |
|---|---|
|  |
|  Minimum volume: >0.2 mL |
Internal: Ambient
Offsite: Refrigerated
Room Temp: 12 hours
Refrigerated: 3 days
Frozen: ≥31 days
| Ordering |
LAB3249
HSVPCR
| Result Interpretation |
| HSV Type 1 | Not detected |
| HSV Type 2 | Not detected |
Interpretation:
Not detected = Negative, no virus detected.
Detected = Positive, virus detected.
Methodology:
This test employs real-time PCR amplification of a Herpes simplex virus-specific conserved genetic target. A positive result should be coupled with clinical indicators for diagnosis. A "Not detected" result for this assay does not exclude Herpes simplex virus involvement in a disease process. This test was developed and its performance characteristics determined by the UCH Clinical Laboratory. It has not been cleared or approved by the U.S. Food and Drug Administration. The FDA has determined that such clearance or approval is not necessary. This test is used for clinical purposes. It should not be regarded as investigational or for research. This laboratory is certified under the Clinical Laboratory Improvement Amendment of 1988 ("CLIA") as qualified to perform high complexity clinical testing.
| Administrative |