
| Specimen Type | Type of Container | Volume of Specimen | Status |
|---|---|---|---|
| Whole blood | Blood Culture Bottles, Anaerobic and Aerobic | See Remarks | Preferred |
| Bone marrow | Aerobic (green) blood culture bottle | 1-2 mL | Preferred |

A single blood collection is considered one draw dispensed into one Aerobic (Green)and one Anaerobic (Purple) bottle. The amount of blood collected depends on the weight of the patient following hospital guidelines:
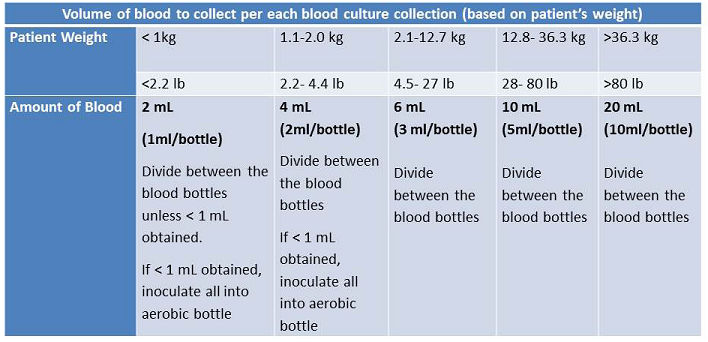
InPatient collection: Please refer to the Guide to Blood Culture Bottle Labeling when labeling samples.
If <1 mL collected, place entire sample in Aerobic (Green) bottle. Bone Marrow: Inoculate entire sample into Aerobic (Green) bottle. Blood culture bottles are incubated 5 days before being finalized. Positive blood culture results are called to physician immediately upon detection. A patient under the age of 4 weeks, in whom a blood culture is being ordered and thus a diagnosis of bacterial sepsis is suspected, should generally receive a full sepsis workup in the ED setting including a lumbar puncture and catheterized urine culture. If this culture is being collected at a lab draw site this recommendation will be faxed and called to the ordering physician's office.
Blood FA
The Blood Culture Identification Panel (Blood FA) is a molecular test that utilizes highly multiplexed PCR to specifically identify 24 organisms and antimicrobial resistance genes within 1-2 hours after the Gram stain reveals organisms in a blood culture bottle. This test allows the preliminary identification of potential pathogens in positive blood cultures. All Blood FA results will be confirmed by culture.
The organisms and the antibiotic resistance markers that are identified by the Blood FA are:
The Blood FA panel will be run on first time positive blood cultures only. It will NOT be run on: body fluids in blood culture media, pathology blood cultures and any bottles that come from outside hospitals.
| Specimen Type | Type of Container | Volume of Specimen | Status |
|---|---|---|---|
| Whole blood | Blood Culture Bottles, Anaerobic and Aerobic | See Remarks | Preferred |
| Bone marrow | Aerobic (green) blood culture bottle | 1-2 mL | Preferred |
| Specimen Type | Type of Container | Minimum Volume |
|---|---|---|
| Whole blood | Blood Culture Bottles, Anaerobic and Aerobic | See Remarks |
| Bone marrow | Aerobic (green) blood culture bottle | 1-2 mL |

A single blood collection is considered one draw dispensed into one Aerobic (Green)and one Anaerobic (Purple) bottle. The amount of blood collected depends on the weight of the patient following hospital guidelines:

InPatient collection: Please refer to the Guide to Blood Culture Bottle Labeling when labeling samples.
If <1 mL collected, place entire sample in Aerobic (Green) bottle. Bone Marrow: Inoculate entire sample into Aerobic (Green) bottle. Blood culture bottles are incubated 5 days before being finalized. Positive blood culture results are called to physician immediately upon detection. A patient under the age of 4 weeks, in whom a blood culture is being ordered and thus a diagnosis of bacterial sepsis is suspected, should generally receive a full sepsis workup in the ED setting including a lumbar puncture and catheterized urine culture. If this culture is being collected at a lab draw site this recommendation will be faxed and called to the ordering physician's office.
Blood FA
The Blood Culture Identification Panel (Blood FA) is a molecular test that utilizes highly multiplexed PCR to specifically identify 24 organisms and antimicrobial resistance genes within 1-2 hours after the Gram stain reveals organisms in a blood culture bottle. This test allows the preliminary identification of potential pathogens in positive blood cultures. All Blood FA results will be confirmed by culture.
The organisms and the antibiotic resistance markers that are identified by the Blood FA are:
The Blood FA panel will be run on first time positive blood cultures only. It will NOT be run on: body fluids in blood culture media, pathology blood cultures and any bottles that come from outside hospitals.
| Specimen Type | Type of Container | Volume of Specimen | Status |
|---|---|---|---|
| Whole blood | Blood Culture Bottles, Anaerobic and Aerobic | See Remarks | Preferred |
| Bone marrow | Aerobic (green) blood culture bottle | 1-2 mL | Preferred |
| Specimen Type | Type of Container | Minimum Volume |
|---|---|---|
| Whole blood | Blood Culture Bottles, Anaerobic and Aerobic | See Remarks |
| Bone marrow | Aerobic (green) blood culture bottle | 1-2 mL |

A single blood collection is considered one draw dispensed into one Aerobic (Green)and one Anaerobic (Purple) bottle. The amount of blood collected depends on the weight of the patient following hospital guidelines:

InPatient collection: Please refer to the Guide to Blood Culture Bottle Labeling when labeling samples.
If <1 mL collected, place entire sample in Aerobic (Green) bottle. Bone Marrow: Inoculate entire sample into Aerobic (Green) bottle. Blood culture bottles are incubated 5 days before being finalized. Positive blood culture results are called to physician immediately upon detection. A patient under the age of 4 weeks, in whom a blood culture is being ordered and thus a diagnosis of bacterial sepsis is suspected, should generally receive a full sepsis workup in the ED setting including a lumbar puncture and catheterized urine culture. If this culture is being collected at a lab draw site this recommendation will be faxed and called to the ordering physician's office.
Blood FA
The Blood Culture Identification Panel (Blood FA) is a molecular test that utilizes highly multiplexed PCR to specifically identify 24 organisms and antimicrobial resistance genes within 1-2 hours after the Gram stain reveals organisms in a blood culture bottle. This test allows the preliminary identification of potential pathogens in positive blood cultures. All Blood FA results will be confirmed by culture.
The organisms and the antibiotic resistance markers that are identified by the Blood FA are:
The Blood FA panel will be run on first time positive blood cultures only. It will NOT be run on: body fluids in blood culture media, pathology blood cultures and any bottles that come from outside hospitals.
| Outpatient Requirements |
| Specimen Type | Type of Container | Volume of Specimen | Status |
|---|---|---|---|
| Whole blood | Blood Culture Bottles, Anaerobic and Aerobic | See Remarks | Preferred |
| Bone marrow | Aerobic (green) blood culture bottle | 1-2 mL | Preferred |

A single blood collection is considered one draw dispensed into one Aerobic (Green)and one Anaerobic (Purple) bottle. The amount of blood collected depends on the weight of the patient following hospital guidelines:

InPatient collection: Please refer to the Guide to Blood Culture Bottle Labeling when labeling samples.
If <1 mL collected, place entire sample in Aerobic (Green) bottle. Bone Marrow: Inoculate entire sample into Aerobic (Green) bottle. Blood culture bottles are incubated 5 days before being finalized. Positive blood culture results are called to physician immediately upon detection. A patient under the age of 4 weeks, in whom a blood culture is being ordered and thus a diagnosis of bacterial sepsis is suspected, should generally receive a full sepsis workup in the ED setting including a lumbar puncture and catheterized urine culture. If this culture is being collected at a lab draw site this recommendation will be faxed and called to the ordering physician's office.
Blood FA
The Blood Culture Identification Panel (Blood FA) is a molecular test that utilizes highly multiplexed PCR to specifically identify 24 organisms and antimicrobial resistance genes within 1-2 hours after the Gram stain reveals organisms in a blood culture bottle. This test allows the preliminary identification of potential pathogens in positive blood cultures. All Blood FA results will be confirmed by culture.
The organisms and the antibiotic resistance markers that are identified by the Blood FA are:
The Blood FA panel will be run on first time positive blood cultures only. It will NOT be run on: body fluids in blood culture media, pathology blood cultures and any bottles that come from outside hospitals.
| Inpatient Requirements |
| Specimen Type | Type of Container | Volume of Specimen | Status |
|---|---|---|---|
| Whole blood | Blood Culture Bottles, Anaerobic and Aerobic | See Remarks | Preferred |
| Bone marrow | Aerobic (green) blood culture bottle | 1-2 mL | Preferred |
| Specimen Type | Type of Container | Minimum Volume |
|---|---|---|
| Whole blood | Blood Culture Bottles, Anaerobic and Aerobic | See Remarks |
| Bone marrow | Aerobic (green) blood culture bottle | 1-2 mL |

A single blood collection is considered one draw dispensed into one Aerobic (Green)and one Anaerobic (Purple) bottle. The amount of blood collected depends on the weight of the patient following hospital guidelines:

InPatient collection: Please refer to the Guide to Blood Culture Bottle Labeling when labeling samples.
If <1 mL collected, place entire sample in Aerobic (Green) bottle. Bone Marrow: Inoculate entire sample into Aerobic (Green) bottle. Blood culture bottles are incubated 5 days before being finalized. Positive blood culture results are called to physician immediately upon detection. A patient under the age of 4 weeks, in whom a blood culture is being ordered and thus a diagnosis of bacterial sepsis is suspected, should generally receive a full sepsis workup in the ED setting including a lumbar puncture and catheterized urine culture. If this culture is being collected at a lab draw site this recommendation will be faxed and called to the ordering physician's office.
Blood FA
The Blood Culture Identification Panel (Blood FA) is a molecular test that utilizes highly multiplexed PCR to specifically identify 24 organisms and antimicrobial resistance genes within 1-2 hours after the Gram stain reveals organisms in a blood culture bottle. This test allows the preliminary identification of potential pathogens in positive blood cultures. All Blood FA results will be confirmed by culture.
The organisms and the antibiotic resistance markers that are identified by the Blood FA are:
The Blood FA panel will be run on first time positive blood cultures only. It will NOT be run on: body fluids in blood culture media, pathology blood cultures and any bottles that come from outside hospitals.
| Overview/Billing |
| Interpretation |
| NCH Lab Only |
| Specimen Type | Type of Container | Volume of Specimen | Status |
|---|---|---|---|
| Whole blood | Blood Culture Bottles, Anaerobic and Aerobic | See Remarks | Preferred |
| Bone marrow | Aerobic (green) blood culture bottle | 1-2 mL | Preferred |
| Specimen Type | Type of Container | Minimum Volume |
|---|---|---|
| Whole blood | Blood Culture Bottles, Anaerobic and Aerobic | See Remarks |
| Bone marrow | Aerobic (green) blood culture bottle | 1-2 mL |

A single blood collection is considered one draw dispensed into one Aerobic (Green)and one Anaerobic (Purple) bottle. The amount of blood collected depends on the weight of the patient following hospital guidelines:

InPatient collection: Please refer to the Guide to Blood Culture Bottle Labeling when labeling samples.
If <1 mL collected, place entire sample in Aerobic (Green) bottle. Bone Marrow: Inoculate entire sample into Aerobic (Green) bottle. Blood culture bottles are incubated 5 days before being finalized. Positive blood culture results are called to physician immediately upon detection. A patient under the age of 4 weeks, in whom a blood culture is being ordered and thus a diagnosis of bacterial sepsis is suspected, should generally receive a full sepsis workup in the ED setting including a lumbar puncture and catheterized urine culture. If this culture is being collected at a lab draw site this recommendation will be faxed and called to the ordering physician's office.
Blood FA
The Blood Culture Identification Panel (Blood FA) is a molecular test that utilizes highly multiplexed PCR to specifically identify 24 organisms and antimicrobial resistance genes within 1-2 hours after the Gram stain reveals organisms in a blood culture bottle. This test allows the preliminary identification of potential pathogens in positive blood cultures. All Blood FA results will be confirmed by culture.
The organisms and the antibiotic resistance markers that are identified by the Blood FA are:
The Blood FA panel will be run on first time positive blood cultures only. It will NOT be run on: body fluids in blood culture media, pathology blood cultures and any bottles that come from outside hospitals.