

| Transport Instructions | |||
| Collection Location | Transport Temperature | Processing Required | Timeframe |
| ED/Inpatient | Room Temperature | None | Specimen must be received by the lab ASAP or within 24 hours of collection |
| Laboratory/Outpatient/Off-Site | Room Temperature | None | Specimen must be received by the lab ASAP or within 24 hours of collection |


| Ordering |
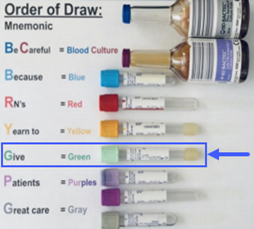
| Collection |

| Transport Instructions | |||
| Collection Location | Transport Temperature | Processing Required | Timeframe |
| ED/Inpatient | Room Temperature | None | Specimen must be received by the lab ASAP or within 24 hours of collection |
| Laboratory/Outpatient/Off-Site | Room Temperature | None | Specimen must be received by the lab ASAP or within 24 hours of collection |



| Result Interpretation |