
Orders must be placed using the Epic Wizard, “COVID-19/FLU/RESP VIRUS PANEL WIZARD.” Restrictions may apply.
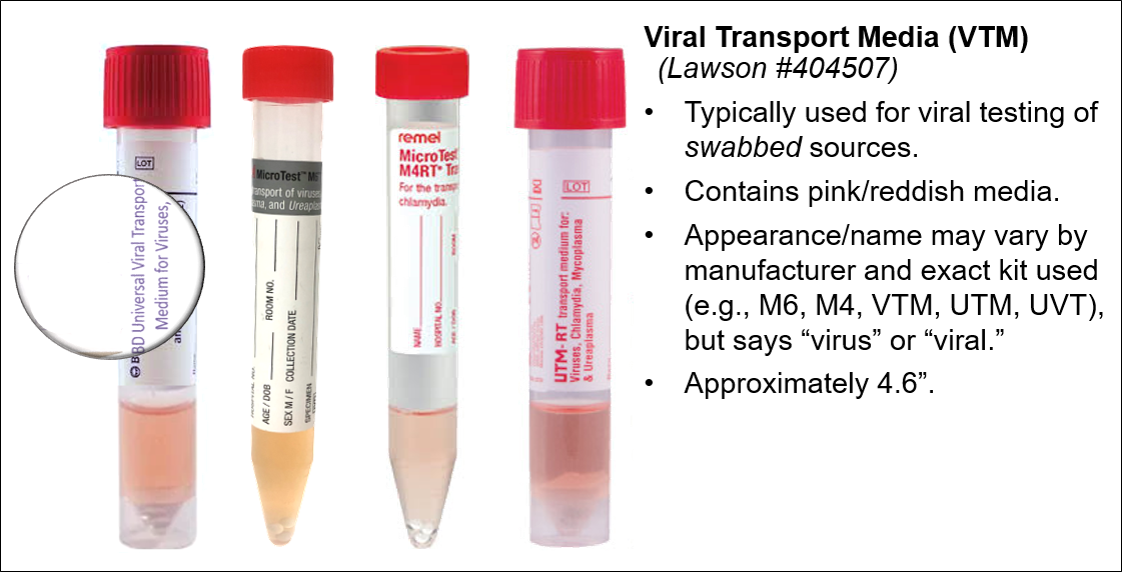
See lower respiratory order for testing on lower respiratory sources.
Internal: Ambient
Offsite: Refrigerated
Room Temp: 4 hours
Refrigerated: 3 days
Frozen: 31 days
Specimen source must be identified on order or requisition.
This test includes qualitative testing for the following:
Adenovirus, Coronavirus 229E, Coronavirus HKU1, Coronavirus NL63, Coronavirus OC43, SARS-COV-2, Human Metapneumovirus, Rhinovirus/Enterovirus, Influenza A, Influenza A H1 2009, Influenza A H1, Influenza A H3, Influenza B, Parainfluenza 1,2,3, and 4, Respiratory Syncytial Virus, Bordetella pararapertussis, Bordetella pertussis, Chlamydia pneumoniae, Mycoplasma pneumoniae.
Same day
Orders must be placed using the Epic Wizard, “COVID-19/FLU/RESP VIRUS PANEL WIZARD.” Restrictions may apply.
| ADENOVIRUS | Not detected |
| CORONAVIRUS 229E | Not detected |
| CORONAVIRUS HKU1 | Not detected |
| CORONAVIRUS NL63 | Not detected |
| CORONAVIRUS OC43 | Not detected |
| SARS-COV-2 | Not detected |
| HUMAN METAPNEUMOVIRUS | Not detected |
| RHINOVIRUS/ENTEROVIRUS | Not detected |
| INFLUENZA A | Not detected |
| INFLUENZA A H1 2009 | Not detected |
| INFLUENZA A H1 | Not detected |
| INFLUENZA A H3 | Not detected |
| INFLUENZA A (NO SUBTYPE DETECTED) | Not detected |
| INFLUENZA B | Not detected |
| PARAINFLUENZA 1 | Not detected |
| PARAINFLUENZA 2 | Not detected |
| PARAINFLUENZA 3 | Not detected |
| PARAINFLUENZA 4 | Not detected |
| RESPIRATORY SYNCYTIAL VIRUS | Not detected |
| B PARAPERTUSSIS | Not detected |
| B PERTUSSIS | Not detected |
| C PNEUMONIAE | Not detected |
| M PNEUMONIAE | Not detected |
| Collection |
Orders must be placed using the Epic Wizard, “COVID-19/FLU/RESP VIRUS PANEL WIZARD.” Restrictions may apply.

See lower respiratory order for testing on lower respiratory sources.
Internal: Ambient
Offsite: Refrigerated
Room Temp: 4 hours
Refrigerated: 3 days
Frozen: 31 days
Specimen source must be identified on order or requisition.
This test includes qualitative testing for the following:
Adenovirus, Coronavirus 229E, Coronavirus HKU1, Coronavirus NL63, Coronavirus OC43, SARS-COV-2, Human Metapneumovirus, Rhinovirus/Enterovirus, Influenza A, Influenza A H1 2009, Influenza A H1, Influenza A H3, Influenza B, Parainfluenza 1,2,3, and 4, Respiratory Syncytial Virus, Bordetella pararapertussis, Bordetella pertussis, Chlamydia pneumoniae, Mycoplasma pneumoniae.
| Ordering |
Same day
Orders must be placed using the Epic Wizard, “COVID-19/FLU/RESP VIRUS PANEL WIZARD.” Restrictions may apply.
| Result Interpretation |
| ADENOVIRUS | Not detected |
| CORONAVIRUS 229E | Not detected |
| CORONAVIRUS HKU1 | Not detected |
| CORONAVIRUS NL63 | Not detected |
| CORONAVIRUS OC43 | Not detected |
| SARS-COV-2 | Not detected |
| HUMAN METAPNEUMOVIRUS | Not detected |
| RHINOVIRUS/ENTEROVIRUS | Not detected |
| INFLUENZA A | Not detected |
| INFLUENZA A H1 2009 | Not detected |
| INFLUENZA A H1 | Not detected |
| INFLUENZA A H3 | Not detected |
| INFLUENZA A (NO SUBTYPE DETECTED) | Not detected |
| INFLUENZA B | Not detected |
| PARAINFLUENZA 1 | Not detected |
| PARAINFLUENZA 2 | Not detected |
| PARAINFLUENZA 3 | Not detected |
| PARAINFLUENZA 4 | Not detected |
| RESPIRATORY SYNCYTIAL VIRUS | Not detected |
| B PARAPERTUSSIS | Not detected |
| B PERTUSSIS | Not detected |
| C PNEUMONIAE | Not detected |
| M PNEUMONIAE | Not detected |
| Administrative |